Why this matters
In industrial free radical polymerizations, product quality, as represented by molecular weight and reduced viscosity, differs with the choice of initiators as well as other variations in the process. By using ACOMP’s real-time data, it is possible to monitor deviations in concentrations, conversion, and molecular weight and correlate to how they impact material properties with different initiators. ACOMP identifies this information at an early stage during the reaction, which can be used to take corrective actions and drive improvements to the chemistry and reaction process, saving tremendous cost and time.
Overview
ACOMP provides a real-time, online analysis of polymerization reactions. This is achieved by continuous sample extraction and characterization as the reaction unfolds, providing users with immediate insight into kinetics and the macromolecular polymer properties such as molecular weight, reduced viscosity, and in some cases composition and polydispersity. This technical note describes how ACOMP can track the properties of polyacrylamide produced with different initiator types and concentrations.
Thermal free radical initiators are commonly used to polymerize monomers like acrylamide. To be industrially useful, an initiator should be relatively stable at room temperature, but it should decompose rapidly at the desired reaction temperature to ensure an optimal reaction rate. In addition to temperature, the decomposition rate (kD) of the initiator will depend on the solvent, monomers and their relative concentrations. In batch free radical polymerizations like that of polyacrylamide, the polymerization roughly follows first-order kinetics. In practice, decomposition is a complicated process which has an impact on the structure of the synthesized polymer molecules.
Reactions with Potassium Persulfate Initiator (KPS)
Adjusting the monomer to initiator molar ratio is a well-known method for controlling end-product molecular weight and viscosity in polymer synthesis. However, the results do not always agree with theoretical computations due to multiple factors in process scale-up. Even when using the same initiator with other conditions nominally held constant, significant variations may occur. Such issues lead to expending tremendous development time on repeating reactions and characterizing end products instead of focusing on improvements and the development of polymers with a target molecular weight, viscosity, composition, etc.
In this note, we compare effects of initiator type and concentration in the synthesis of polyacrylamide, a specialty polymer with many industrial applications. A commonly-used initiator is potassium persulfate, KPS. By increasing the monomer to initiator ratio, theoretically, the end-product should have a higher molecular weight. Tables 1 & 2 show parameters for reactions run with 1.5 wt% and 1.0 wt% KPS, respectively. These reactions were run with 15g monomer in 480 +/- 2 ml solution with 0.224 +/- 0.001g of KPS for Table 1 and 0.150 +/- 0.001g KPS for Table 2.
| Reaction | Time to 65˚C | N2 Pre-Purge Time |
|
1 |
24 min | 41 min |
| 2 | 40 min | 62 min |
| 3 | 39 min | 55 min |
| 4 | 38 min | 56 min |
| 5 | 60 min | 79 min |
Table 1: Polyacrylamide reaction condition table (1.5wt% KPS initiator)
| Reaction | Time to 65˚C | N2 Pre-Purge Time |
|
1 |
43 min | 61 min |
| 2 | 33 min | 50 min |
| 3 | 34 min | 52 min |
| 4 | 34 min | 52 min |
| 5 | 34 min | 51 min |
Table 2: Polyacrylamide reaction condition table (1.0wt% KPS initiator)
The ACOMP system continuously extracts a polymer sample from the reactor and, using its light scattering detector, measures the weight-average molecular weight in real time. The molecular weight is an absolute molecular weight from physical theory, and it does not depend on correlations.
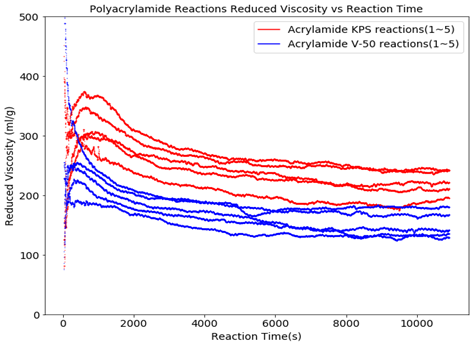
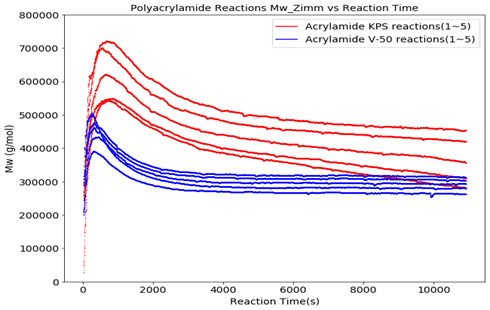
Figure 1 shows the effect of the initiator ratio change on the molecular weight. Except for the initiator concentration, all polymerizations were nominally carried out under the same conditions, but even at the same initiator level in the same recipe, it is obvious that other unknown factors significantly affect the molecular weight. Still, it can be observed that overall at 1.0 wt% KPS the polymer, somewhat counterintuitively, exhibits a steeper molecular weight decrease over the reaction time of about 1.5 hours. Figure 2 shows the reduced viscosity for the 1 wt% KPS reactions.
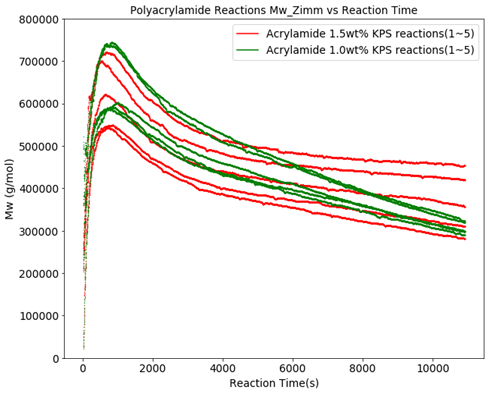
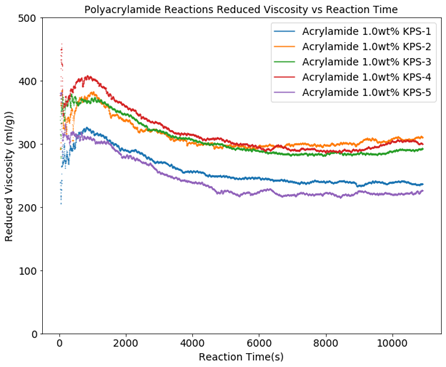
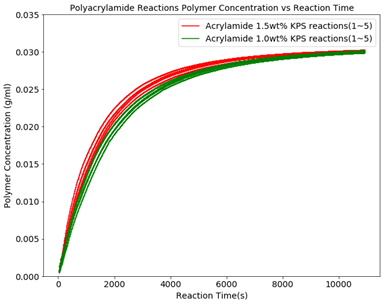
Figure 3 shows the polymer concentration in the reactor for the KPS reactions at 1.5 and 1.0 wt%. This graph clearly shows that the concentration of initiator has an impact on the polymerization reaction kinetics, however, the reproducibility at the same nominal concentration is rather poor. This results in the molecular weight and reduced viscosity trend changing during the reaction and impacts the end products from batch to batch as seen in Figures 1 and 2.
Recipe Selections and the Effects of Differing Initiators
For a given polymer, there could be various choices for the initiator. Manufacturers consider many aspects when selecting an initiator, such as the cost, difficulty of storage, and shelf life. Without knowledge of the initiator’s effect on the reaction kinetics, stringent product quality control would be difficult due to the long characterization cycle (characterization of the end-product from offline QC analysis), low statistical information (information only from aliquots), and overall analysis cost and time. The example presented below demonstrates how ACOMP greatly simplifies and shortens the process of identifying an appropriate initiator.
Table 3 shows conditions of five reaction runs conducted with another initiator. V-50 is a highly active water-soluble azo polymerization initiator available from FujiFilm Wako Chemicals. These reactions were run on a standard recipe of 15g monomer and 0.225g +/- 0.001g V-50 in 480 +/- 2 ml of solution but with small variations in temperature ramp and nitrogen pre-purge.
| Reaction | Time to 65˚C | N2 Pre-Purge Time |
|
1 |
37 min | 54 min |
| 2 | 38 min | 58 min |
| 3 | 38 min | 57 min |
| 4 | 40 min | 57 min |
| 5 | 37 min | 53 min |
Table 3: Polyacrylamide reaction condition table using V-50 1.5wt%
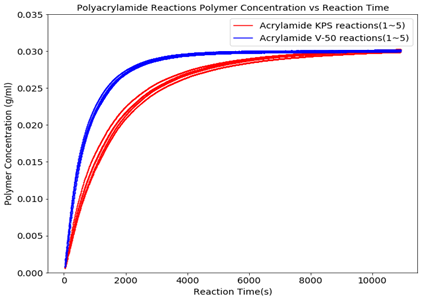
Figure 4 shows the reactor polymer concentration trends of 3wt% acrylamide reactions conducted with KPS and V-50 with the same monomer to initiator molar ratio. It is clear that the kinetics of the polymerizations with KPS exhibit a noticeable discrepancy between reactions. This may be from the KPS having a lower energy barrier to form free radicals. Under non-isothermal conditions, the temperature at different spots within the reactor could lead to different decomposition rates. Additionally, as the KPS gets consumed, the pH of the reaction media decreases, further affecting the decomposition rate throughout the reaction (1,2).
Figure 4 shows that the reactions conducted with V-50 have a better reproducibility. Not only does the V-50 initiator have a higher decomposition energy, but it also efficiently forms free radicals that quickly interact with acrylamide monomers and rapidly disperse in the reaction system (3). Also, the reaction kinetics are much faster when using V-50 as the initiator.
Figures 5 and 6 show the molecular weight and reduced viscosity comparison between the V-50 and the KPS initiated polymerizations. Figure 6 clearly shows the contrast between the molecular weight evolution during the reaction and the end-product differences. Potential factors for the differences in molecular weight and reduced viscosity include the oxygen level in the reactor at the beginning of the reaction and the initiator efficiency once the viscosity has built up in the reactor during the polymerization process.
Conclusion
Product quality, as represented by molecular weight and reduced viscosity, differs significantly according to initiator choice. Random process variations from batch to batch also affect product properties, even when using the same recipe. By using ACOMP, it is possible to monitor real-time deviations in concentration and conversion and measure the resulting material’s macromolecular properties such as molecular weight and reduced viscosity. ACOMP identifies this information at an early stage during the reaction and can help drive improvements to the chemistry and reaction process, saving tremendous cost and time. For more information on the quality control process in polyacrylamide reactions, download the ACOMP QC technical note.
References
- “Thermal decomposition of potassium persulfate in aqueous solution at 50°C in an atmosphere of nitrogen in the presence of acrylonitrile monomer”, [J] Applied Polymer Science, https://doi.org/10.1002/app.1988.070350604
- “Solution Polymerization of acrylamide using potassium persulfate as initiator: kinetic studies, temperature and pH dependence”, [J] European Polymer Journal, 37(2991) 1507-1510
- “Rapid Decomposition of a Cationic Azo-Initiator Under Microwave Irradiation”, [J] Applied Polymer Science, https://doi.org/10.1002/app.32409


